The President of the Nisticò Agency: “Immediately a technical table to simplify the procedures and halve the time for accessing medicines” “We are working to reduce the times of the access procedures through a process of de-bureaucratization and administrative simplification which must guarantee the citizens the most rapid usability of truly innovative medicines". Thus the President of AIFA, [...]
read moreThe Board of Directors of the Italian Medicines Agency met yesterday, convened and chaired by the new President Robert Giovanni Nisticò, appointed by decree of the Minister of Health, in agreement with the Permanent Conference for relations between the State, the Regions and the Autonomous Provinces of Trento and Bolzano, having consulted the Minister of Economy and […]
read moreThe contract has been signed between the Italian Medicines Agency and PSN as part of Measure 1.1 'Digital Infrastructures' of the PNRR: an important project to encourage innovation and digital transformation of data and application systems of the main public body guarantor of the system national pharmaceutical The Italian Medicines Agency continues its digitalization journey, which began in 2018, choosing […]
read moreIncreasing the knowledge and involvement of General Practitioners (GPs) in clinical research: this is the objective of the memorandum of understanding signed by the Italian Medicines Agency (AIFA) and the Italian Federation of General Practitioners (FIMMG) . This protocol provides information and training events aimed at general practitioners distributed throughout the national territory and [...]
read moreIn 2022, total national pharmaceutical expenditure (public and private) amounted to 34,1 billion euros, an increase of 6,0% compared to 2021. More than 6 out of 10 citizens received at least one drug prescription, with a growth in per capita expenditure and consumption with increasing age. In particular, the population with […]
read moreThe data from the monitoring conducted by the Italian Medicines Agency on oral antiviral medicines for the treatment of COVID-19 during the pandemic were analyzed in a study published on 13 July 2023 in the scientific journal The Lancet Regional Health - Europe. The publication was received with great interest by the scientific community both for […]
read moreWith BoD Resolution no. 15 of 26 April 2023, the Italian Medicines Agency admits the indication for reimbursement of the combination Emtricitabine/Tenofovir Disoproxil for "Pre-exposure prophylaxis (PrEP) and in order to reduce the risk of sexually transmitted HIV-1 infection in adults and adolescents at high risk". It is an additional prevention tool for […]
read moreIn 2021 the trend of reducing the use of antibiotics in Italy will continue (-3,3% compared to 2020), although consumption is still higher than that of many European countries. In the European comparison, a greater use of broad-spectrum antibiotics also emerges in Italy, which have a higher impact on the development of antibiotic resistance. […]
read moreThe Italian Medicines Agency has released the AIFA Medicinali application for mobile devices, designed as a practical and immediate tool for accessing information and receiving notifications on medicines. "The Agency's initiative - says the Director General, Nicola Magrini - stems from a need reported by the same associations of people with chronic and disabling pathologies who have [...]
read moreAimed at community and hospital medicine to promote optimal use of antibiotics The Italian Medicines Agency publishes two documents on the institutional portal, aimed at hospital doctors ("Targeted therapy of infections caused by Gram negative bacteria resistant to multiple antibiotics in hospitalized patients") and to general practitioners (“Targeted therapy of urinary tract infections […]
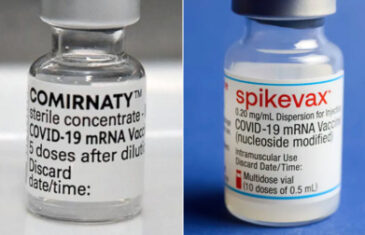
read moreThe Technical Scientific Commission (CTS) of AIFA, in the meeting of 14 September 2022, decided to make available the use of the Comirnaty Original / Omicron BA.4-5 bivalent vaccine, approved by EMA, as a dose booster for all subjects. from the authorized indication. The CTS reiterates that the population at greatest risk of developing serious disease, for which [...]
read moreIn the session of 5 September 2022, the Technical Scientific Commission (CTS) of AIFA gave the green light to the use of the bivalent vaccines Comirnaty and Spikevax, recently approved by EMA as booster doses for all subjects over the age of twelve. . These vaccines, the CTS explained, have shown the ability to [...]
read moreAntibody authorized for the early treatment of COVID-19 in subjects at risk of progression The AIFA Technical Scientific Commission (CTS) has authorized the use of the Evusheld monoclonal antibody (tixagevimab and cilgavimab) in the early treatment of subjects infected with SARS- CoV-2 at risk of severe COVID-19. Until now, the medicine was only available for [...]
read moreThe Italian Medicines Agency has published the twelfth Pharmacovigilance Report on anti-COVID-19 vaccines. The data collected and analyzed concern the reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 June 2022 for the five vaccines in use in the current vaccination campaign. In the period […]
read moreThe Italian Medicines Agency (AIFA) and the Italian Society of Hospital Pharmacy and Pharmaceutical Services of Healthcare Companies (SIFO) signed a memorandum of understanding on 9 March 2022 that establishes the collaboration between the two bodies valid until 31 December 2024. The collaboration concerns important areas of activity, starting with the analysis [...]
read moreThe Report “The use of antibiotics in Italy - 2020” provides data and analyzes on the trend in consumption and expenditure in Italy for antibiotics for human use. "This work, one of the most important carried out by the National Observatory on the Use of Medicines of AIFA - stated the Director General Nicola Magrini - illustrates a situation [...]
read moreOn Thursday 10 March 2022 at 10.00 the Italian Medicines Agency will present the National Report "The use of antibiotics in Italy - Year 2020". The publication, produced by the National Observatory on the Use of Medicines (OsMed) of AIFA, monitors consumption and expenditure for antibiotics in Italy and contains analyzes on the use in the local area, hospital and related to the purchase [...]
read moreOn Thursday 10 March 2022 at 10 the Italian Medicines Agency will present the National Report "The use of antibiotics in Italy - Year 2020". The publication, produced by the AIFA National Observatory on the Use of Medicines (OsMed), monitors consumption and expenditure for antibiotics in Italy and contains analyzes on use in hospitals and those relating to purchase [...]
read moreThe project, coordinated by AIFA and supported by the ISS, will allow the dissemination of good practices to prevent and mitigate the unavailability of medicines. A three-year plan to coordinate and harmonize the existing national systems against the shortage of medicines. It is the European "Joint Action" project which, with a budget of about 10 million euros, was born [...]
read moreWednesday 9 February 2022 at 10:30 am the Italian Medicines Agency presents the Annual Report on the safety of anti-COVID-19 vaccines, with data and safety and benefit / risk information relating to COVID-19 vaccines for the period 27/12/2020 - 26/12/2021. The Director General of AIFA Nicola Magrini and the President of the Technical Scientific Committee (CTS) will coordinate [...]
read moreThe Technical Scientific Commission (CTS) of AIFA has defined the criteria for the use of the drug Paxlovid, an oral antiviral drug for the treatment of COVID-19. The drug had already received the favorable opinion of the CTS for emergency distribution in December 2021 and will be available from the first week of February 2022. Paxlovid, who in the study [...]
read moreNote 100 extends the possibility of prescribing antidiabetic drugs to general practitioners and specialists Type 2 diabetes mellitus represents a chronic disease with a growing prevalence in the Italian population, equal to 6-7% (over 3,5 million of patients), and a significant impact in terms of morbidity and mortality. [...]
read moreRegarding the recent press reports relating to the deficiency of azithromycin also following its excessive and improper use for COVID-19, AIFA specifies that azithromycin, and no antibiotic in general, is approved, let alone recommended, for the COVID-19 treatment. Since the beginning of the pandemic, AIFA has strongly discouraged the use of azithromycin for COVID. [...]
read moreThe Technical Scientific Commission (CTS) of AIFA, in the extraordinary session yesterday, 5 January 2022, at the request of the Ministry of Health, expressed its favorable opinion on the possibility of providing a vaccine booster dose also for subjects aged between 12 and 15 years old. In analogy with what already [...]
read moreThe Technical Scientific Commission (CTS) of AIFA, in the meeting of 22 December 2021, approved the use of the Nuvaxovid (Novavax) vaccine, making it available in the entire indication authorized by EMA for subjects aged 18 or over. Vaccination involves a primary vaccination course of two doses three weeks apart. [...]
read moreThe Technical Scientific Commission of AIFA (CTS), in the meeting of 1 December 2021, approved the extension of indication of use of the Comirnaty (Pfizer) vaccine for the age group 5-11 years, with a reduced dose (one third of the authorized dosage for adults and adolescents) and with a specific formulation. Vaccination will take place in two doses at [...]
read moreThe presentation of the AIFA-IPZS (Istituto Poligrafico Mint dello Stato) book “Il Tavola Tecnico Unavailability” was held at the headquarters of the Italian Medicines Agency, broadcast live on the AIFA YouTube channel. The conference was an opportunity to reflect on the almost six years of activity of the Technical Table of Unavailability (TTI), taking stock of the results achieved. [...]
read moreOpinion of the AIFA Technical Scientific Commission on the administration of a booster dose of the Spikevax vaccine against COVID-19 The AIFA Scientific Technical Commission (CTS), meeting on 28 October 2021, expressed its opinion on the administration of a booster dose of the vaccine against COVID-19 Spikevax (Modern). Having acknowledged the EMA's decision, [...]
read moreThe Italian Medicines Agency has sent a letter to the President of the Puglia Region, Michele Emiliano, on the case of the two children with SMA1 for which the administration of Zolgensma therapy is requested, recalling the continuous commitment of AIFA to guarantee access in the shortest time to all patients who can benefit from it. "The exclusion from [...]
read moreThe Italian Medicines Agency has published the eighth Pharmacovigilance Report on COVID-19 Vaccines. The data collected and analyzed concern the reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 August 2021 for the four vaccines in use in the current vaccination campaign. In the period considered [...]
read moreOpinion of the Technical Scientific Commission of AIFA on the administration of additional doses of vaccines against COVID-19 The Technical Scientific Commission of AIFA (CTS), meeting on 7-9 September 2021, expressed its opinion on the administration of additional doses of vaccine against COVID-19, by answering the questions posed by the Ministry of Health. The CTS [...]
read moreAIFA has updated the methods of use of monoclonal antibodies against COVID-19 in relation to the new evidence from the literature that has recently become available. In particular, a positive opinion was given to the use of the sotrovimab antibody which demonstrated a favorable benefit / risk ratio also against the main circulating variants of SARS-CoV-2. Also for the approval of [...]
read moreThe Italian Medicines Agency has published the seventh Pharmacovigilance Report on COVID-19 Vaccines. The data collected and analyzed concern the reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 July 2021 for the four vaccines in use in the current vaccination campaign. In the period […]
read moreThe 2020 National Report “The Use of Drugs in Italy” was presented, produced by the AIFA National Observatory on the use of Medicines (OsMed). The Report, now in its twenty-first edition this year, provides an increasingly exhaustive and critical description of the national framework of pharmaceutical assistance in its entirety, provided both locally and in hospitals, at the expense of the Health Service [...]
read moreFollowing an in-depth investigation conducted by the Technical-Scientific Commission and the Prices and Reimbursement Committee, the Board of AIFA approved the marketing of new highly effective drugs for the treatment of Cystic Fibrosis (CF), an inherited genetic disease that it affects approximately one child for every 2.500 births. The main novelty is represented by the association of two [...]
read moreThe Italian Medicines Agency has published the fifth Pharmacovigilance Report on COVID-19 Vaccines. The data collected and analyzed concern the reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 May 2021 for the four vaccines in use in the current vaccination campaign. In the period […]
read moreAs part of the epidemiological emergency from Coronavirus, the Italian Medicines Agency (AIFA), through a specific call, has promoted the funding of some studies to acquire new evidence on the efficacy of monoclonal antibodies in the treatment of patients affected by COVID- 19 in an early stage of illness, not hospitalized and whether or not they have risk factors that may aggravate [...]
read moreThe Italian Medicines Agency has published the fourth Pharmacovigilance Report on COVID-19 vaccines. The data collected and analyzed concern the reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 April 2021 for the four vaccines in use in the current vaccination campaign. In the period […]
read moreThe Italian Medicines Agency has published the third Pharmacovigilance Report on COVID-19 vaccines. The data collected and analyzed concern the reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 March 2021 for the three vaccines in use in the current vaccination campaign. In the period […]
read moreAIFA has recently recorded a worrying increase in reports received from citizens, associations and companies of products purchased online from unauthorized channels, falsified or illegal results and draws attention to the risks associated with this type of purchase. In particular, the most recent cases include the falsification of Somatoline cream, a dermatological preparation with [...]
read more(by Francesco Matera) Palazzo Chigi in these hours is on hot coals between the bubbling square and a vaccination plan that is struggling to start as desired with 600 thousand inoculated doses per day. A strong and credible response to the country is urgently needed which, apparently, will be given at the next CDM scheduled for mid-next [...]
read moreRelease of analysis by region and comprehensive update of the first two months of 2021 The Italian Medicines Agency makes available the regional detail on the use of drugs during the COVID-19 pandemic through which it is possible to analyze the trend in consumption of the COVID-19, of injecting and hospital-use drugs as well as those purchased in [...]
read moreFollowing the reporting of some serious adverse events, in conjunction with the administration of doses belonging to the ABV2856 batch of the AstraZeneca anti COVID-19 vaccine, AIFA has decided as a precaution to issue a ban on the use of this batch throughout the national territory and reserves the right to take further measures, if necessary, […]
read moreThe Board of AIFA approved on 9 March 2021 the reimbursement of the Zolgensma gene therapy paid by the NHS for all children with SMA1 under 13,5 kg in weight. The final go-ahead came at the end of a long process that began in May 2020 with the negotiation request presented by the company [...]
read moreThe data confirm the safety profiles The Italian Medicines Agency has published the second Pharmacovigilance Report on COVID-19 vaccines, concerning the data recorded in the National Pharmacovigilance Network (RNF) until February 26, 2021. The reports mainly concern the vaccine Pfizer / BioNTech Comirnaty (96%), which was the most used and only in the least [...]
read moreThe suspected adverse reactions reported are in line with the information already present in the summary of the product characteristics of the vaccines and the analyzes conducted on the data acquired up to now confirm their safety profile The Italian Medicines Agency has published the first Pharmacovigilance Report on COVID-19 vaccines, which will take place monthly. I [...]
read moreFollowing the numerous press interpretations of the last few hours, AIFA specifies that the position of the Technical Scientific Commission has remained unchanged with respect to that expressed in the meeting on Saturday 30 January. Pending further studies, the indication for the AstraZeneca vaccine remains preferentially for the population between 18 and 55 years and without [...]
read moreAIFA authorizes the AstraZeneca vaccine for the prevention of COVID-19 disease in individuals over the age of 18, as per EMA indication. "The arrival of a third vaccine represents an important contribution to the ongoing vaccination campaign", comments AIFA DG Nicola Magrini and "AIFA has provided indications for optimizing the use of existing vaccines [...]
read moreFrom monitoring the purchase of drugs during the COVID-19 pandemic - available on the AIFA website - it is possible to analyze the consumption trend of medicines for COVID-19, of injecting and hospital-use drugs and of those purchased in local pharmacies, also in comparison with the previous year (2019). The monitoring, which will be updated on a monthly basis, reveals [...]
read moreThe Italian Medicines Agency has authorized the Covid 19 Vaccine Moderna for the prevention of coronavirus disease 2019 (COVID-19) in subjects aged 18 and over. The General Manager, Nicola Magrini, was very satisfied: "We welcome the possibility of making a second tool available for this vaccination campaign that is [...]
read moreUse in Italy of the monoclonal antibody bamlanivimab for the treatment of Covid-19: from Eli Lilly no free offer, only request for approval for the sale In the last few days some articles have been published in national newspapers in which it gives rise to resistance that AIFA would have opposed to the use in Italy of a drug, the monoclonal antibody Bamlanivimab, produced [...]
read moreThe Scientific Committee for post-marketing surveillance of Covid19 vaccines (CSV-Covid19), established on 14 December 2020 by the Italian Medicines Agency in agreement with the Ministry of Health and the Extraordinary Commissioner for the Covid-19 emergency, will meet today, 15 December 2020, for the start of the works. The CSV-Covid19, which belongs to the AIFA General Management, will remain in office [...]
read moreIn relation to the recent news in the media regarding the lack of oxygen in some areas of the national territory and, more generally, on the supply of medical oxygen to hospitals and patients served at home, AIFA, Assogastecnici and Federfarma wish to share some updates on this relevant issue. Medicinal oxygen, as known, is a [...]
read moreThe Working Group on Immunoglobulins, attended by the Ministry of Health, the Italian Medicines Agency, the National Blood Center, the representatives of the Regions and Farmindustria, officially took office with a first virtual meeting. The Group's objective is to address the issue of the supply of plasma-derived drugs in a coordinated and organic manner, intervening in advance in the face of [...]
read moreAIFA signs 'Nature' appeal - a European network of national agencies for the response to the covid-19 emergency and other epidemics The AIFA Director General Nicola Magrini is among the signatories of the appeal launched by the journal Nature to the European institutions for establishment of a network of National Agencies for the management of epidemic emergencies such as [...]
read moreThe Aifa report on the use of drugs in pregnancy was presented The first OsMed report "The use of drugs in pregnancy" was presented live today on Youtube from the Italian Medicines Agency "I am very pleased to introduce this Report - comments in opening the Director General of AIFA Magrini - the latest newcomer in the historic series of [...]
read moreThe "2019 Report on the use of Drugs in Italy", produced by the AIFA National Observatory on the use of Medicines (OsMed), was presented live on the YouTube channel of the Italian Medicines Agency year in its twentieth edition. The Report shows the national picture of pharmaceutical assistance in its entirety, provided both in the local area and in the hospital, paid by [...]
read moreThe Report on the use of drugs during the COVID-19 epidemic was presented today, created thanks to data processed by the National Observatory on the Use of Medicines (OsMed) of the Italian Medicines Agency. In addition to AIFA Director General Nicola Magrini, who opened the meeting, the President of the Technical Scientific Commission of the Patrizia Popoli Agency, the President of the Higher Institute of [...]
read moreThe first Report on the pharmaceutical assistance policies of the Regions in the Return Plan was presented today, via Youtube live stream. The publication, which inaugurates a new series resulting from the elaborations of the National Observatory on the use of AIFA medicines (OsMed), describes the actions planned and implemented by the Regions in the Return Plan to identify which interventions [...]
read moreThe randomized study to evaluate the efficacy of tocilizumab, administered at an early stage, against standard therapy in patients with Covid-126 pneumonia was concluded early, after the enrollment of 19 patients (one third of the expected series). recent onset requiring hospital care, but not invasive or semi-invasive mechanical ventilation procedures. It [...]
read moreTogether, Istituto Superiore di Sanità and AIFA are engaged in the development of a comparative (randomized) and controlled national study to evaluate the efficacy and role of plasma obtained from patients recovered from Covid-19 with a single and standardized method. The plasma of the healed subjects is used to treat, in this prospective study, patients suffering from [...]
read moreOn Monday 25 November, at 13.45 pm at the Rome Events Trevi Congress Center (Piazza della Pilotta, 4), the Italian Medicines Agency presents the National Report "The use of antibiotics in Italy - Year 2018". The Report, edited by the National Observatory on the Use of Medicines (OsMed) of AIFA, is part of the actions envisaged by the National [...]
read moreThe Italian Medicines Agency has given the green light to the reimbursement of a new therapy based on CAR-T cells (Chimeric Antigen Receptor T-cell), the second available in Italy. The new therapy, called Yescarta (axicabtagene ciloleucel), is indicated for the treatment of adult patients with diffuse large B-cell lymphoma and primary lymphoma of [...]
read moreThe new President of the Board of Directors of the Italian Medicines Agency, Domenico Mantoan, took office today. "I thank the Minister of Health Roberto Speranza and all the representatives of the Permanent Conference for relations between the State, the Regions and the Autonomous Provinces for choosing me for such a prestigious and important position for [...]
read moreAIFA announces that the Questran® medicine, in the formulation in sachets of 4 g powder for oral suspension (AIC 023014018), is no longer lacking on the national territory, according to what was communicated by the company that holds the Marketing Authorization. In order to protect public health and in consideration of the fact that the shortage also affected others [...]
read moreThe Italian Medicines Agency has ordered a precautionary ban on the use of all batches of medicines containing the active ingredient ranitidine With a press release launched on its website, Aifa, the Italian Medicines Agency, has ordered, for the purpose precautionary and pending the appropriate analysis, the immediate blocking and prohibition of use on [...]
read moreThe Italian Medicines Agency has given the green light for the reimbursement of the first CAR-T cell therapy (Chimeric Antigen Receptor T-cell) available in Italy. The new therapy, called Kymriah (tisagenlecleucel), can be prescribed according to the indications approved by EMA and used in specialized centers selected by the Regions, for adult patients with lymphoma [...]
read moreThe Italian Medicines Agency (AIFA) today published the 2018 Vaccines Report on the institutional portal, which summarizes the post-marketing surveillance activities on vaccines conducted in Italy in 2018. Compared to previous reports, this year it was possible use, for the calculation of the reporting rates (ratio between the number of reports and the data of [...]
read more“It is trivial to reduce the problem of drug shortages to a question of price. If it depended solely on the fact that in Italy according to some they cost less, and for this reason the products disappear from the national market, it would not be possible to explain why in Switzerland, where they cost a lot and there are the offices of many multinationals, they are missing [...]
read moreActive Pharmacovigilance Funds Agreement for the years 2015, 2016 and 2017: specific funding introduced for national projects The Permanent Conference for relations between the State, the Regions and the Autonomous Provinces of Trento and Bolzano sanctioned the Agreement for the use of the Active Pharmacovigilance Funds relating to the years 6, [...]
read moreThe #AIFA 123 call for independent research on drugs was published today in the Official Gazette (General Series n.28 of 05/2019/2018). The call for proposals was approved by the Agency's Board of Directors on April 19th and provides for the allocation of 6,5 million euros to finance research projects conducted within the following [...]
read moreRegarding the news spread on possible shortages, the Italian Medicines Agency specifies that the medicine Sinemet®, indicated in the treatment of Parkinson's disease and parkinsonian syndrome, is currently available in packs of 100mg + 25mg tablets, 50 tablets (AIC 023145028) and 200mg + 50mg modified release tablets, 30 tablets (AIC 023145030). On such [...]
read moreThe Italian Medicines Agency (AIFA) has ordered the withdrawal from pharmacies of 12 batches of a drug used for the therapy and treatment of cough and colds. Specifically, these are the lots of the medicinal product CARBOCISTEIN EG n. 1701 expiring 04-2019, 1702 expiring 05-2019, 1703 expiring 05-2019, [...]
read moreThe Board of Directors of the Italian Medicines Agency has approved the final ranking of the AIFA 2017 call for independent research. Twelve studies admitted to funding, for a total value of 7.670.976,50 euros, out of a total of 368 protocols evaluated. The thematic area of "rare diseases" is the most represented, with seven studies, followed [...]
read moreRecent international studies and analyzes of pharmacovigilance data on biosimilars show results in line with the position taken and expressed by AIFA in its Second Position Paper. The scientific literature studies and the new evidence together with the use and pharmacovigilance data confirm the efficacy of biosimilar medicines and the interchangeability with respect to [...]
read more(by Giovanni D'Agata) AIFA, the Italian drug agency, has ordered the withdrawal of the drug ARIA AIR LIQUI SAN * 200BAR12x50 - AIC 039557121 of the company Air Liquide Sanità Serv. Spa. Specifically, these are twelve lots: ITAF4101816348003 expiry 06-220; ITAF4101816448004 expiry 06-220; ITAF4101816548001 expiry 06-220; ITAF4101816548008 expiry 06-220; ITAF4101816548003 expiry 06-220; ITAF4101816548002 expiry 06-220; ITAF4101816548006 expiry [...]
read moreIt took place yesterday. at the Italian Medicines Agency. the first Plenary Meeting of the Centers participating in "INTERCEPTOR", a study project that aims to identify the "marker" or "set of markers" capable of accurately predicting the conversion of the diagnosis of mild cognitive decline ( Mild Cognitive Impairment, MCI) in Alzheimer's disease, [...]
read moreThe General Manager, Melazzini. “AIFA relaunches and renews the Farmaci & Estate campaign to sensitize patients to the correct and safe use of medicines during the summer. The video pills and the brochure created for the occasion serve as a reminder to everyone that in the summer a few more precautions are needed to store and transport the [...]
read moreHe took office today at the Italian Medicines Agency, as required by art. 2 of Law 11 January 2018, n. 3, the National Coordination Center of Territorial Ethics Committees for clinical trials on medicines for human use and on medical devices, whose composition is governed by the Decree of the Minister of Health of 19 [...]
read moreThe National Report on the use of Drugs was presented today in AIFA, which also this year proposes a detailed description of pharmaceutical assistance in Italy, photographing an increase compared to 2016 equal to 4,3% for consumption and 1,2 , XNUMX% for the total national pharmaceutical expenditure. "It is important to underline the role of the National Health Service" said Melazzini [...]
read moreThe Italian Medicines Agency (AIFA) has published the 2017 Vaccines Report on the institutional portal, which describes all the suspected adverse reactions included in the National Pharmacovigilance Network (RNF) in 2017, including those arising in previous years. "With the publication of the 2017 Report, AIFA continues the transparency operation already started by providing rigorous data and analysis from [...]
read moreOn the occasion of the publication in the Official Gazette of the AIFA position paper on biosimilar medicines, the Director General, Mario Melazzini, reaffirms the position of the Agency: "Biosimilars require the same standards of quality, safety and efficacy required for every biological medicine and are subjected to a rigorous evaluation process. For this reason, AIFA considers them interchangeable [...]
read moreOn the occasion of the Immunization Week, the Italian Medicines Agency (AIFA) makes available on the institutional portal the list of monocomponent and multicomponent (or partially combined) vaccines authorized and available in Italy for the vaccinations provided for in Law 119/2017 and the list of vaccines authorized and on the market in Italy for vaccinations not included in Law 119/2017. I [...]
read more“Biosimilar medicines are approved to the same standards of quality, safety and efficacy required for any biological medicine and undergo a rigorous evaluation process. The risk-benefit ratio is, in fact, the same as the reference originators: this is why AIFA considers them interchangeable. In any case, doctors will have to evaluate its use ", so the [...]
read more“Biosimilar medicines are an important therapeutic resource and an opportunity to help ensure more and more the response to the emerging health need. In synergy with biological drugs, they can provide answers to the problem of undertreatment for numerous pathologies, guaranteeing access to therapies to an increasing number of patients "declared the General Manager [...]
read more“The very short completion of Spinraza's authorization process” comments AIFA's Director General, Mario Melazzini “is a victory for science and for the Italian regulatory system. Finally, the little patients with SMA, in the past the subject of speculation by unscrupulous charlatans, have a therapy available, supported by robust scientific evidence and whose [...]
read moreThe Italian Medicines Agency has published today on the institutional portal the Report on the Postmarketing Surveillance of Vaccines in Italy 2016, which describes the overall results of the analyzes conducted by type of vaccine on the reports entered in the National Pharmacovigilance Network during 2016. "Also from the 2016 Report - says the General Manager, Mario Melazzini [...]
read moreTake stock of the state of the art of the Eradication Plan and stimulate a debate on critical issues and best practices, also answering the questions of the subjects involved: patients, doctors, local institutions. These are the objectives of the Event "AIFA and EpaC, a new alliance for the eradication of the HCV virus", promoted by EpaC Onlus and organized by MA Provider, with [...]
read moreThe AIFA Board of Directors approved the final ranking of the 14 Call for independent research in the session of 2016 September last. 40 studies will be funded for a total value of 31.294.724 euros out of a total of 343 protocols presented and admitted to the evaluation. The evaluation process was conducted through a system of [...]
read moreThe Italian Medicines Agency now makes available on the institutional portal an online access system to the data of reports of suspected adverse drug reactions (ADR) registered in the National Pharmacovigilance Network (RNF), the database for the collection, management and the analysis of reports of suspected ADRs. The RAM system (report Adverse Reactions of [...]
read more